Carry out incubations in a humidified chamber to avoid tissue drying out which will lead to non specific binding and high background staining.
Immunofluorescence protocol frozen section.
Sections can be stored in a sealed slide box at 80 c for later use.
Cover sections with 4 formaldehyde diluted in warm 1x pbs.
Paraffin and frozen sections reagents can be applied manually by pipette or this protocol can be adapted for automated and semi automated systems if these are available.
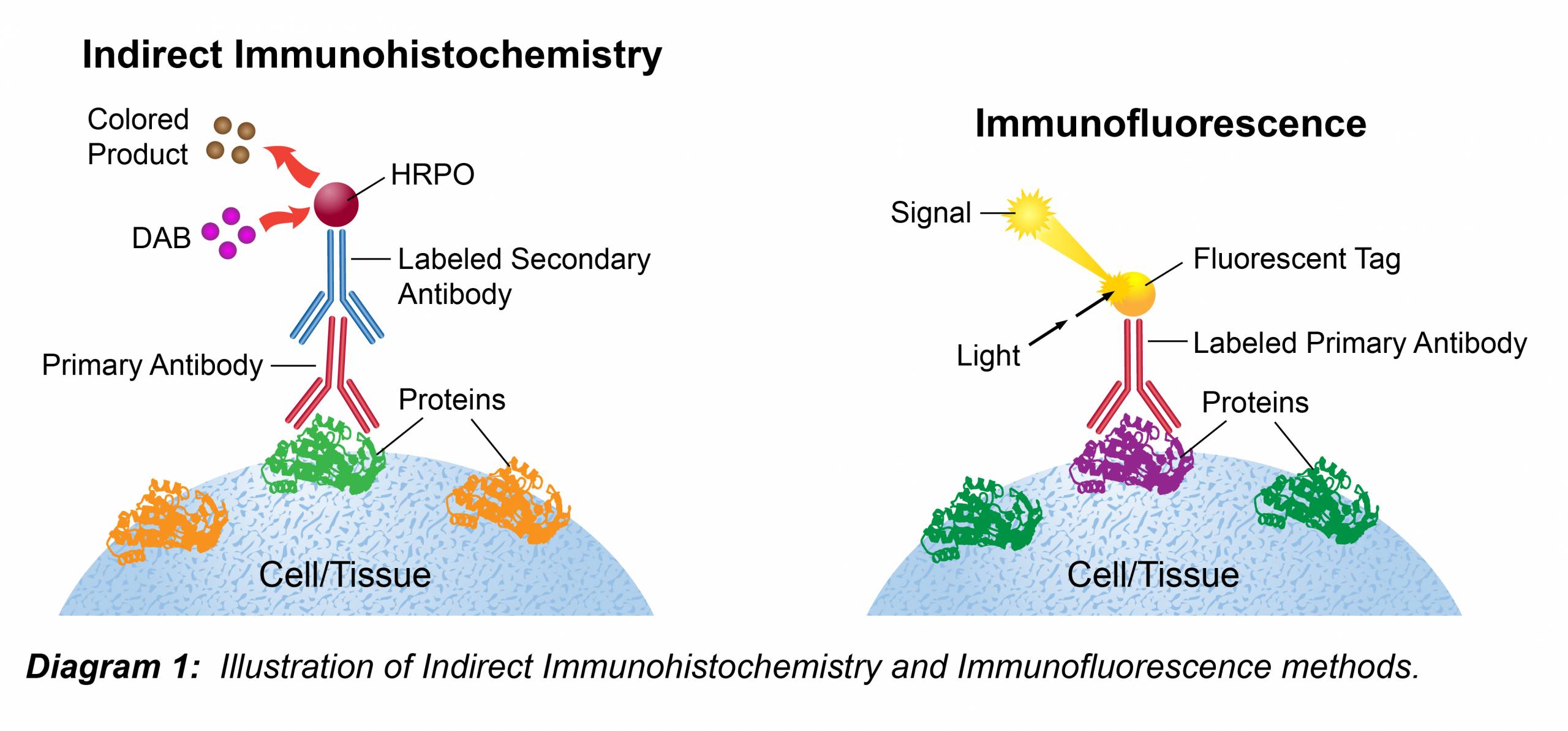
Immunofluorescence is commonly used to determine the cellular or tissue localization of a protein of interest.
Brigitte arduini version 1 2015 mar 23.
For fresh unfixed frozen tissue fix immediately as follows.
Immunofluorescence on frozen sections.
This protocol is also suitable for 40µm free floating.
Section the frozen tissue block into a desired thickness typically 5 10 µm using the cryotome.
Immunofluorescence can also be used as a qualitative measure of protein expression.
Nagy gertsenstein vintersten and behringer ed.
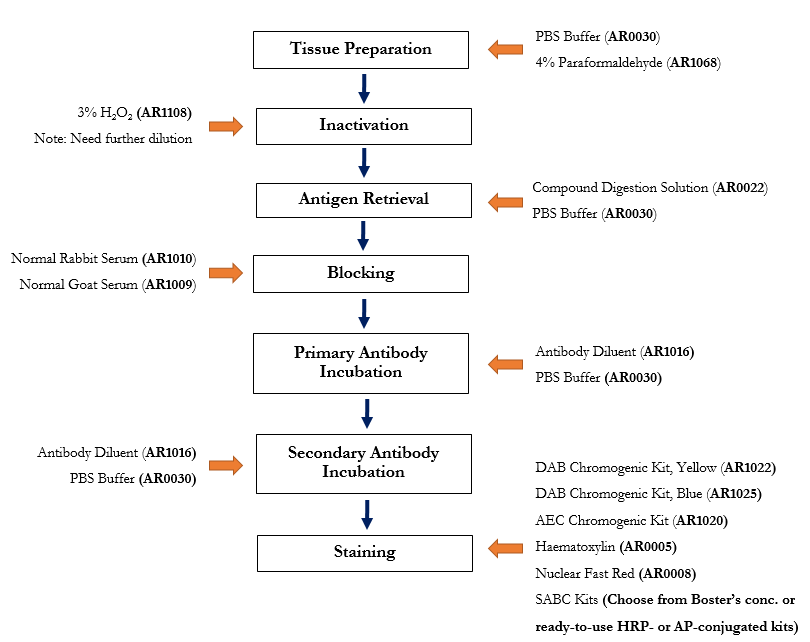
The following immunohistochemistry ihc protocol has been developed and optimized by r d systems ihc icc laboratory for fluorescent ihc experiments using frozen tissue samples.
Allow sections to fix for 15 min at room temperature.
Tissue preparation perfusion and fixation note.
Icc and if video protocol.
Immunocytochemistry and immunofluorescence protocol related fluorescence.
Immunofluorescence on frozen tissue sections bio protocol.
For fixed frozen tissue proceed with immunostaining section c.
Direct vs indirect if.
Microscope slides pre coated.
Snap frozen fresh tissues in liquid nitrogen or isopentane pre cooled in liquid nitrogen embedded in oct compound in cryomolds.
Modified from manipulating the mouse embryo 3.
This ihc protocol provides a basic guide for the fixation cryostat sectioning and staining of frozen tissue samples.
Place the tissue sections onto glass slides suitable for immunohistochemistry e g.
The fluorescent immunohistochemistry immunofluorescence protocol below is intended for the fluorescent visualization of protein expression in frozen tissue sections.
See cryoprotection and processing of embryonic tissue protocol.
Store frozen blocks at 80 ºc.
Materials phosphate buffered saline pbs 1x paraformaldehyde pfa 4 see support protocol 1.
Annexin v labeled with alexa fluor 488 in frozen rat placenta section by ihc immunohistochemistry.
Store slides at 80 ºc until needed.
Protocol for immunofluorescent staining of mouse frozen sections tissue.
Dry the tissue sections overnight at room temperature.